pH is a chemical property of water
Neutral pH is a Property of Pure water
Pure water pH is the starting point of the entire pH system. In fact, pH is a chemical property of pure water and is standardized in terms of pure water.
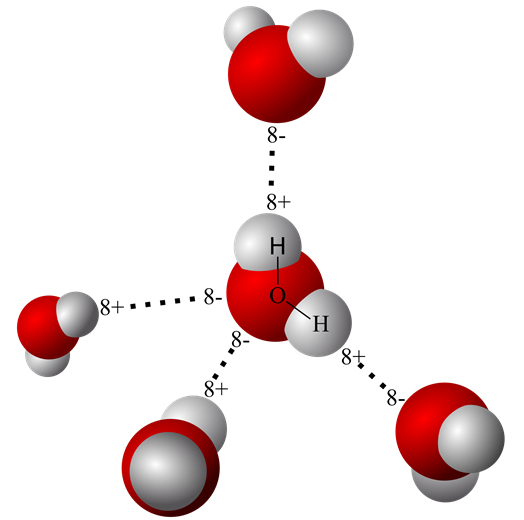
An earlier post on this site titled “Water’s Chemistry Governs Physiology” describes the origin of the water molecule’s oppositely charged geometric ends. It also explains how two hydrogen nuclei with a single proton each share their electron with oxygen to form water. The hydrogen electrons are pulled so far away from their proton in a water molecule that sometimes, even in pure water one of the protons escapes the water molecule leaving behind an hydroxide ion [OH– ].
Demonstration of free hydrogen ion [H+] in pure water was obtained by experimental measurements. Careful quantification revealed that only a small fraction of liquid water molecules reversibly breaks spontaneously.
The formula for this reversible chemical process is:
H2O ↔ [H+] (an acid) + [OH– ] (a base)
There are scientific data showing that the loose proton, [H+], attaches itself to the oxygen end of an adjacent water molecule forming a hydronium ion [H3O+] rather than floating free as a proton.
However, because protons readily move among water molecules and can reunite with the basic [OH– ] they are represented in most equations as just [H+]. pH is defined as the concentration of [H+] in a water solution. That is, pH defines how many [H+] are present per liter of water. The pH of pure water is 7.00, and that turns out to be close to an optimal concentration of [H+] in an environment of biologic molecules.
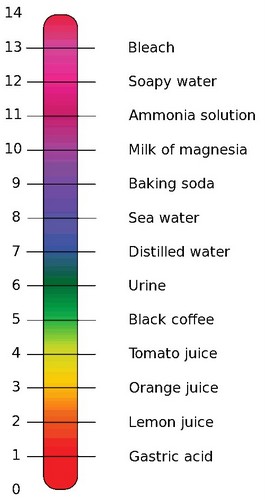
pH range
The pH range of values is 0 – 14. Any pH value greater than 7.00 is said to be a basic pH or alkaline. Any pH below the 7 of pure water is said to be an acidic pH. Because of the way pH is defined, the number 7 means that there are 10-7 moles of [H+] in a liter of pure water. It also means that there are more [H+] in a solution that is pH 3 than in a solution that is pH 7, because 10-7 is a much smaller number than 10-3.
A more detailed explanation of the number scale for pH can be found in my book “Physiology: Custom-Designed Chemistry.” To take a look inside the book for free click here.
pH of body fluids
The pH of body fluids varies from system to system. But, why is it important how many [H+] float around in body fluids? The primary reason is that these acids are likely to randomly break any chemical bond that they get near. And the body only wants bonds broken for a purpose not at random. When a bond breaks in a large biologic molecule, it causes a change in the molecule’s shape. Shape is very important to biologic molecules. When their shape alters, they can no longer get their job done.
Notice in the scale pictured above that the gastric fluids of the stomach have a very acidic pH. This acid condition in the stomach is created for the very purpose of breaking apart large protein molecules in food. Urine also has a pH outside the narrow range of most body fluids, because it accumulates excess [H+] secreted by the kidney. Both the stomach and the urinary bladder have special anatomical features that protect them from acid pH.
There are many contributors, both acidic and alkaline, to the final pH of human body fluids, and they are difficult to measure separately. Yet, pH balance is tightly regulated in the human body. For example, there are multiple layers of regulatory control for balancing blood pH to keep it in the range of 7.35 – 7.45. They include a fast response – blood buffers and lung respiration rate – supplemented by the kidney’s slow but steady removal of [H+].
Further Reading
Buffering Body Alkalinity and Acidity
Do you have questions?
Please put your questions in the comment box or send them to me by email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you find this article helpful share it with your fellow students or send it to your favorite social media site by clicking on one of the buttons below.
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.