Physiology of body compartments
If you are taking courses in anatomy and physiology at the same time, you may be a little puzzled by what your instructor means when talking about body compartments. Large anatomical compartments, such as the abdominal and thoracic cavity are much easier to visualize than the body fluid compartments and other microscopic compartments described in physiology by their biochemistry.
Body fluid compartments
In physiology the easiest type of compartments to begin with are body fluid compartments. The body fluid compartments are easy to overlook, because they are missing in your lab’s anatomic models, dead bones, and even your formalin fixed cadavers. Yet, in a living body they are a major anatomic feature that serves as the platform for whole body communication.
Water, H2O, is the primary component of the body fluid compartments. There are two major fluid compartments, the extracellular compartment and the intracellular compartment. About 60% of body water is within cells, the intracellular compartment. The remainder of the body’s water is found around cells, that is in the interstitial fluid, in blood plasma, lymph, cerebrospinal fluid, gastrointestinal secretion, aqueous humor of the eye, sweat, urine, and in the peritoneal and plural cavities. The barriers between fluid compartments are the plasma membranes of various cells types.
Ion channels
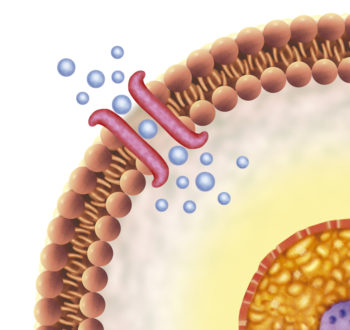
Channels of communication are needed for the body’s fluid compartments to interact with each other. Information proceeds in both directions across cell membranes through ion channels. For maximum control of information transfer between compartments, it is necessary that the ion channels are small and that they open and close in a non-random fashion.
Ions, atoms carrying a positive or negative charge, are key messengers that travel between fluid compartments when small doors, or channels, in cellular plasma membranes open. Ions are atoms that have either lost one or more of their electrons or have acquired an extra one from another atom. Because of their charge ions are soluble in water but not in the cell membrane lipids and require a watery channel for passage through cell membranes.
Ions exist because some atoms have loosely attached electrons that can be stolen by atoms with larger nuclei that contain more protons. The atom that gets the extra electron(s) has a total negative charge and is called an anion. The atom that is missing an electron or two has a total positive charge and is called a cation. Both are collectively referred to as ions. The abundance of particular ions varies in different fluid compartments.
Ions, moving from one fluid compartment to another create a flow of chemical electricity. The pattern of electrical current creates a message. The major ionic players in creating electrical flow through the membrane of nerve and muscle cells are chloride [Cl–], sodium [Na+], potassium [K+], and calcium [Ca++].
Gated ion channels
Membrane ion channels are said to be gated. Gated ion channels only open under particular circumstances. When membrane channels open to allow passage of ions, the direction an ion moves is determined by its concentration in the intracellular and extracellular fluid. Ions always move through watery compartment from where their concentration is high to where their concentration is low. This process is called diffusion.
Whether an ion can pass through an open channel depends upon the protein structure of that channel. All transmembrane ion channels are proteins. The size of the membrane channel and its ability to strip water molecules away from an ion influence the the passage of the ion. Ion channel properties are different enough that channels are named for the ion that they allow to pass. In physiology, you will study potassium channels, sodium channels, chloride channels, calcium channels, etc.
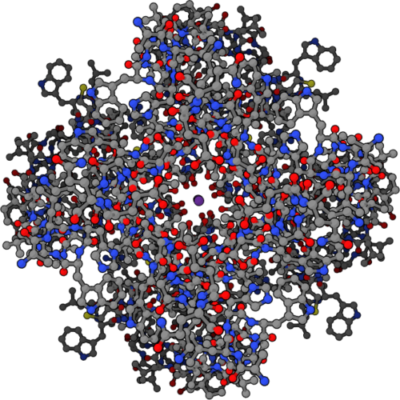
Four part symmetrical structure of potassium ion channel with potassium ion at center, Public Domain/Wikimedia Commons
An additional factor regulating an ion’s diffusion through an open membrane channel is the presence of an electrical charge along the inside and outside surface of the membrane. The physiologic name for the combined force of concentration gradient and electrical gradient across cell membranes on diffusion of fluid compartment ions is the electrochemical gradient.
Ions channels play a role in a large array of physiologic processes. Ion channels for sodium [Na+], potassium [K+], and calcium [Ca++] and chloride [Cl–]are important to contraction of skeletal, smooth and heart muscle. But, they have many other responsibilities as well in normal body function. Other ions of major importance include hydrogen [H+], zinc [Zn++], iron [Fe++/Fe+++], and magnesium [Mg++].
Continue to think of these small charged atoms as chemical and electrical messengers as you study the physiology of cell signaling between body fluid compartments. Doing so will help make the biochemistry of physiologic processes become less mysterious.
Do you have questions?
Please put your questions in the comment box or send them to me by email at DrReece@MedicalScienceNavigator.com. I read and reply to all comments and email.
If you find this article helpful share it with your fellow students or send it to your favorite social media site by clicking on one of the buttons below.
Further reading
3 Simple Secrets to Learning Physiology
Neurons: Where Does Their Electricity Come From?
Margaret Thompson Reece PhD, physiologist, former Senior Scientist and Laboratory Director at academic medical centers in California, New York and Massachusetts is now Manager at Reece Biomedical Consulting LLC.
She taught physiology for over 30 years to undergraduate and graduate students, at two- and four-year colleges, in the classroom and in the research laboratory. Her books “Physiology: Custom-Designed Chemistry”, “Inside the Closed World of the Brain”, and her online course “30-Day Challenge: Craft Your Plan for Learning Physiology”, and “Busy Student’s Anatomy & Physiology Study Journal” are created for those planning a career in healthcare. More about her books is available at https://www.amazon.com/author/margaretreece. You may contact Dr. Reece at DrReece@MedicalScienceNavigator.com, or on LinkedIn.
Dr. Reece offers a free 30 minute “how-to-get-started” phone conference to students struggling with human anatomy and physiology. Schedule an appointment by email at DrReece@MedicalScienceNavigator.com.


This is cool!
i was looking for such easy task from a long time it will help me in increasing my knowledge. thanks a lot.
Keep up the great piece of work, I read few content on this site and I conceive that your blog is rattling interesting and has sets of superb information.
nice!!!
I was recommended this web site by my cousin. I’m not sure whether this post is written by him as no one else know such detailed about my difficulty. You’re incredible! Thanks!
I am trying to include things that my students have struggled with in the past. What do you find most difficult about this course? What other topics would you like me to write about?
This web site certainly has all the information I wanted concerning this subject and didn’t know who to ask.
Pingback:Molecule Structure Hydrogen - Chemical Literature